VaxMaX Updates
VaxMaX has been updated to include a set of reports available to all providers to support planning and reporting of orders, administration, and redistributions within the network. Providers should now see a ‘Provider Reports’ tab in their VaxMaX account. Reports include some of the following views:
- Supply & demand over time and by provider site,
- Compliance report comparing the calculated inventory (shipments +/- redistributions minus administrations) and vaccine finder inventory inputs
- Requests/allocations/orders by provider site, and
- Demographics report that shows who providers have vaccinated.
For any questions about VaxMaX and its functionality, please visit the VaxMaX help website as a resource for reference guides and tutorial videos.
Virginia COVID-19 Vaccination Updates
VaccineFinder now redirects to a new website, vaccines.gov, which is available in English and Spanish, and has high accessibility standards.
- The website provides information about nearby locations offering vaccines.
- In addition to the website, people in the U.S. are also now able to utilize a text messaging service, available in both English and Spanish where they text their ZIP code to 438829 (GETVAX) or 822862 (VACUNA) to find three locations nearby that have vaccines available.
- Please share this resource with your patients and community.
VDH has launched a Vaccinate with Confidence webpage for providers with resources for inspiring vaccine confidence and webinar recordings focused on the topic. Please visit the site and share with your networks and colleagues.
The Pfizer-BioNTech COVID-19 vaccine could be authorized by the Food and Drug Administration (FDA) for use in adolescents aged 12 to 15 years by next week.
- According to the manufacturer, clinical trial data showed that its vaccine was at least as effective in adolescents aged 12 to 15 years as it was in adults.
- FDA is reviewing these data and could add an amendment covering that age group to the vaccine’s existing emergency use authorization (EUA) by next week.
- If you anticipate providing COVID-19 vaccines to children under 16 years of age, VDH encourages you to attend our May 18 expert panel focused on pediatric COVID-19 vaccination.
As a reminder, the following updates were made to the Moderna COVID-19 vaccine EUA on April 1 regarding its storage and handling.
- Frozen Vaccine: Moderna vials can now be stored frozen between -50o to -15oC (-58o to 5oF). This is an increased range from the original temperatures. This new, wider temperature range is consistent with temperature requirements for other recommended vaccines stored in the freezer.
- Refrigerated Vaccine/Unpunctured Vials: Vials may be stored between 8° to 25°C (46° to 77°F) for a total of 24 hours. This is an increase from 12 hours.
- Punctured Vials: After the first dose has been withdrawn, the vial should be held between 2° to 25°C (36° to 77°F) for up to 12 hours. Vials should be discarded 12 hours after the first puncture. This is an increase from 6 hours.
Best Practice Spotlight
Slips, trips, and falls (STFs) can lead to broken bones, concussions, or even life-threatening conditions and death in serious cases. COVID-19 vaccination sites should implement mitigation strategies to prevent STFs of both their patients and clinic staff.
To prevent slips, trips and falls in vaccination sites, it is important to evaluate key exposure risks that may be present and implement controls that may result in a reduction in injury frequency and severity. Zurich, an insurance company, has developed the following recommendations to mitigate STFs when identifying and preparing COVID-19 vaccination sites:
- When possible, choose an area with surfaces with higher slip resistance, such as non-slippery natural stone, broom-finished concrete and carpet versus hard, smooth surfaces.
- Review surface conditions as you consider areas to be used for vaccination sites. For example, raised or recessed sidewalk edges or curbing, potholes in parking lots, painted surfaces, loose carpeting, loose or broken tiles, holes or pits on the surface, or unusual wear could lead to STFs.
- Ice, water, liquids, powders, grease or any substances that could be tracked into the building or spilled in the vaccination area could cause STFs. Consider the use of walk-off mats at building entrances to minimize the tracking of water or other substances that increase the risk of STFs. Review surfaces periodically to identify potential spills that could cause a slip/fall incident. Also, control the types of and quantities of liquids in the vaccination centers to minimize potential spills. Soft drinks, water and vaccines may spill if mishandled and create a slip hazard.
- Review the room/spaces to be used along with the paths of travel to/from the rooms to assure that level changes can be navigated by all the persons expected to utilize the spaces.
- Obstructions, such as extension cords, hoses, portable freezer storage, concrete posts, and temporary storage/holding areas can contribute to the likelihood of an STF. Consider using cord guards or, lacking that, adhesive tape to secure obstructions and minimize the potential trip hazard.
Upcoming Events
- Mondays, Wednesdays, and Fridays, 4 - 5 pm: VDH | Office Hours for Providers (including VaXMaX assistance)
- May 12, 2 pm: HRSA | COVID-19 Coverage Assistance Fund: Provider Webcast
- May 18, 12:15 - 1:15 pm: VDH | Pediatric COVID-19 Vaccination Panel
- May 19, 2 - 3 pm: New Mexico Immunization Coalition (NMIC), NMDOH and Comagine Health | Coexisting with COVID--Strategies for Catching up on Routine Vaccines
- May 20, 2 - 4:30 pm: National Adult and Influenza Immunization Summit (NAIIS) | Maintaining Influenza Prevention During the Ongoing COVID-19 Pandemic
Additional upcoming events
Additional upcoming events can be found here.
Helpful Resources
One way CDC is contributing to vaccination efforts is by promoting the work happening in communities across the country through COVID-19 Vaccine Community Features. The latest features include:
- Esperanza Health Centers Work to Deliver COVID-19 Vaccines | CDC
- Southern New Jersey Approach to Overcoming the COVID-19 Pandemic | CDC
The National Institute for Occupational Safety and Health (NIOSH) recently released a label that can be printed and applied to alternative sharps disposal containers when FDA-cleared sharps disposal containers are scarce. An accompanying webpage is available on CDC’s website here.
The below Wastage Reporting Table provides guidance to determine if a dose should be reported as waste. Wastage does not negatively impact a provider but is simply a means for accounting for inventory. For this reason, please report all wasted doses in the Vaccine Wastage Reporting Tool.
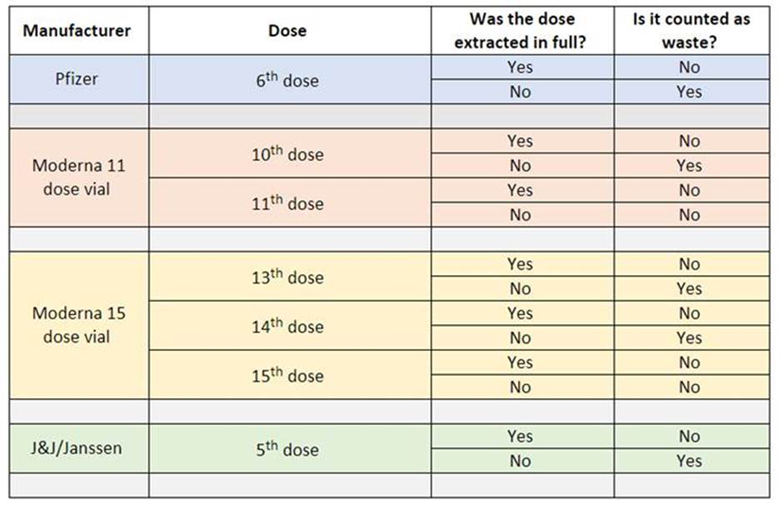

Information about the COVID-19 vaccination program is changing frequently. This newsletter will offer regular updates to providers who have submitted an intent to vaccinate or signed the CDC provider agreement within Virginia.
VDH COVID-19 Vaccination Response: Healthcare Professionals Website

