
COVID-19 Updates for Virginia
September 20, 2021
Dear Colleague:
Thank you for your continued partnership in responding to the COVID-19 pandemic. Please visit the Virginia Department of Health (VDH) website for current clinical and public health guidance, epidemiologic data, and other information. Updates on the following topics are included in this correspondence:
- Back-to-School Resources and Reminders
- CDC’s Health Alert Network (HAN) Health Advisory about Ivermectin
- Update on Monoclonal Antibody Treatment for COVID-19
- Virginia Immunization Information System Requires All Immunizations January 2022
Back-to-School Resources and Reminders
With K-12 schools back in session, we want to make sure healthcare providers are aware of key COVID-19 resources and updates. The Virginia Department of Health’s (VDH) and the Virginia Department of Education’s Interim Guidance for COVID-19 Prevention in Virginia PreK-12 Schools summarizes overarching principles for keeping schools safely open and provides a step-by-step guide for operational decisions.
On September 9, VDH posted a new supplemental guide called Clarification of VDH K-12 Close Contact Definitions and Quarantine Periods. VDH allows an exception to the close contact definition for students in indoor and outdoor K-12 settings, including school buses that meet certain criteria. A student who was within 3 to 6 feet of an infected student is not considered a close contact as long as both students wore well-fitting masks the entire time.
This document also details the options for quarantine. Close contacts who are not fully vaccinated should stay home (quarantine) after an exposure. A 14-day quarantine is the safest option, but two shorter options may be considered if a 14-day quarantine causes undue hardship that may reduce compliance. For more information on these options and the potential increases in post-quarantine transmission risk, please also review the CDC Science Brief. Please note the data described in this report are prior to Delta being the predominant variant in the United States. The Centers for Disease Control and Prevention (CDC) has not changed guidance on quarantine duration based on the emergence of the Delta variant but will continue to monitor the evidence and revise guidance if needed.
VDH updated the Guideline: When Should a Child Stay Home from School and/or Child Care? and the 1-page Algorithm for Evaluating a Child with COVID-19 Symptoms or Exposures to align with the CDC recommendations. Template letters for schools to use when notifying people about close contact exposure, a COVID-19 case, or an outbreak were also revised to request that parents bring the letter to their healthcare provider if they seek COVID-19 testing or related care. As a reminder, VDH maintains extensive General FAQs (see “Schools [K-12]” section) and Vaccination FAQs (see “Children” and “Healthcare Providers” sections). Other materials are available on VDH’s K-12 Education website.
CDC’s Health Alert Network (HAN) Health Advisory: Rapid Increase in Ivermectin Prescriptions and Reports of Severe Illness Associated with Use of Products Containing Ivermectin to Prevent or Treat COVID-19
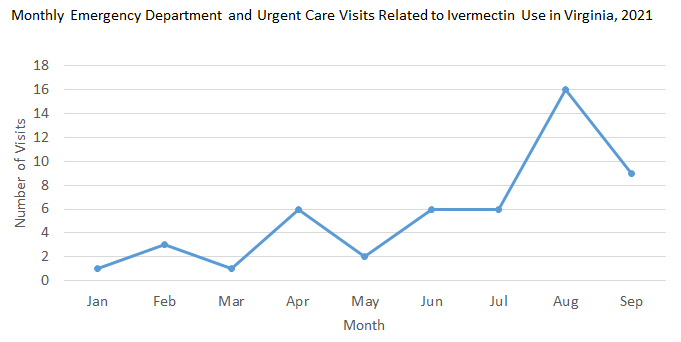
In this August 26 health advisory, CDC emphasizes that ivermectin is not recommended for COVID-19 prophylaxis or treatment because there are insufficient effectiveness data from clinical trials and observational studies. Ivermectin is an FDA-approved medication to treat parasitic infections or skin conditions (e.g., onchocerciasis, intestinal strongyloidiasis, lice, rosacea). Veterinary formulations, which are available over the counter for parasitic infections in animals, are not intended for humans. Ivermectin is safe when used for approved conditions and as prescribed by a healthcare provider. If people take ivermectin and develop symptoms, they should seek immediate medical attention or call the poison control center (1-800-222-1222). Signs and symptoms include gastrointestinal effects (nausea, vomiting, abdominal pain, and diarrhea), headache, blurred vision, dizziness, fast heart rate, and low blood pressure. The figure below shows a recent increase in emergency department and urgent care center visits related to ivermectin use in Virginia. Monthly visits in September have already exceeded visits in each month this year, except August.
Update on Monoclonal Antibody Treatment
On September 13, 2021, the U.S. Department of Health and Human Services (HHS) changed the way the monoclonal antibodies REGEN-COV and Bamlanivimab/Etesevimab (“Bam/Ete”) will be ordered from HHS and distributed. Under this new system, HHS will allocate a specific number of doses of REGEN-COV and Bam/Ete weekly to each state. Per HHS, allocation will be based on the number of COVID-19 cases and hospitalizations in the state. VDH will now control the monoclonal antibody (mAb) product and its distribution. VDH will determine the sites that receive mAbs and the quantity of product they will get. Once VDH reviews and approves an order from a mAb administration site, AmerisourceBergen (the distributor) will ship the product to the recipient.
For the week starting September 13, 2021, Virginia has been allocated 1,370 doses of REGEN-COV and 160 doses of Bam/Ete. HHS informed all states they cannot order extra doses of monoclonal antibodies in excess of their weekly allocation.
In response to the above, VDH has quickly set up a process where mAb administration sites can place orders with VDH. Initially, there will be two groups – sites that have an immediate need for REGEN-COV and/or Bam/Ete and those that don’t. An email with a link to an online Monoclonal Antibody Order Form was sent to all administration sites on Thursday, September 16, 2021. Sites were instructed to order only one week’s worth of medication. VDH reviewed the forms and attempted to fill each order with the type and quantity of product requested. On or about Monday, September 20, 2021, a second email will be sent to mAb administration sites describing the ongoing ordering process.
Please note this process will be refined over time into a more regular routine. We ask for and appreciate your patience and cooperation as we transition to this new paradigm. If you have questions, please email the general monoclonal antibody mailbox at mabs_requests@vdh.virginia.gov.
Providers Required to Report All Immunizations to Virginia Immunization Information System Starting January 2022
As a COVID-19 Vaccination Program Provider, all COVID-19 vaccine administrations must be reported to the Virginia Immunization Information System (VIIS) per the CDC Agreement all participating providers sign. Reporting to VIIS has been invaluable, allowing public health to identify pockets of need across the Commonwealth, clinicians to determine previous immunization history, and for people to retrieve their immunization records when they have lost their vaccination cards.
VIIS, however, is not just for COVID-19 vaccines. VIIS is a free statewide registry system that combines immunization histories for persons of all ages from both the public and the private sector. The goal of VIIS is to support individuals, families and clinicians in making the best healthcare decisions by providing a statewide, readily accessible and reliable Immunization Information System. Many of you already use VIIS regularly in your day-to-day operations to make the best healthcare decisions with your patients. VIIS has a website you can log into to review immunization history for your patients or add immunizations to their records. It also has the ability to connect directly to your electronic medical record system. VIIS just completed a major system upgrade this year and future enhancements are planned for 2022 to improve its efficiency, functionality, and user experience.
As a reminder, pursuant to Chapter 211 of the 2021 Special Session I, as of January 1, 2022, any health care provider, as defined in §32.1-127.1:03, in the Commonwealth that administers immunizations shall report such patient immunization information to the Virginia Immunization Information System (VIIS) pursuant to §32.1-46.01.
If you are not already actively submitting immunization administration data to VIIS or unsure if you are, please contact our team as soon as possible. Please reach out VIISInfo@vdh.virginia.gov and a VIIS Trainer will contact you.
The Provider Enrollment Process consists of the following:
- Discussion with a VIIS Trainer.
- Identify main points of contact for VDH at your organization.
- What access levels will your employees have.
- Complete and submit a registration in the Virginia Electronic Registration for Immunization Programs (VERIP) system.
- Attend a live webinar VIIS training session.
- Access to VIIS will be granted.
As we onboard healthcare providers across the state, we appreciate your patience during this time while the VIIS team members work to process all enrollment requests.
Thank you for all your continued efforts to protect Virginians from COVID-19. If you have questions about COVID-19, please contact your local health department.
Sincerely,
M. Norman Oliver, MD, MA
State Health Commissioner
