
Virginia Mpox Updates

As of August 19, 2025, 22 mpox cases have been reported to VDH this year. To compare, Virginia had 27 cases in 2024. A concerning increase occurred this July with 13 of the 22 cases being reported. Of 2025 cases, 12 were in the Northern region, five in Central, four in Eastern, and one in Southwest. Among this year’s cases with available information, six of 19 (32%) were in people who recently traveled. This suggests that most infections were locally acquired. Five of 21 (24%) cases were in people with HIV. Thirteen of 19 (68%) were in people who were not vaccinated. As in recent years, most of this year’s cases occurred in adult males.
To date, all mpox cases in Virginia have been caused by Clade II monkeypox virus. This is the strain responsible for the global outbreak that began in 2022. Large Clade I and Clade II outbreaks are occurring, including in Central and Eastern African and in West Africa.
While our local health departments received this update and the below recommendations, we ask our valued community partners to continue to:
-
- Educate the public about mpox prevention and vaccination
- Include mpox in appropriate outreach and education services
- Refer eligible clients to their local health department for vaccination and relevant services
- Download, use, and share VDH mpox digital and print resources
Contact Diana Prat, Deputy Director, Division of Disease Prevention, at diana.prat@vdh.virginia.gov, or 804-864-7961, with any questions about DDP mpox efforts or for additional assistance.
DDP Data Dashboards
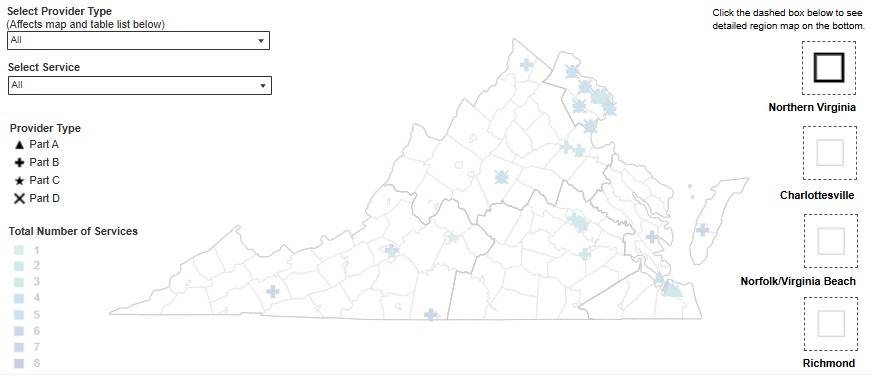 In collaboration with the Division of Informatics and Information Systems (DIIS), DDP has released a new interactive dashboard for Ryan White services. The dashboard is a map of service providers throughout the Commonwealth. It allows users to filter providers by region or service type (part A, B, C, or D). Pop out maps are provided on the map to provide detailed views of areas with higher provider density. You can find the interactive map on the Ryan White Resources page. The webpage includes a description of the different Ryan White parts beneath the map. Please note that this dashboard does not take the place of a service directory and does not contain detailed contact information for agencies. If you see corrections or changes needed to the dashboard, please contact Rivkah Meder at rebecca.meder@vdh.virginia.gov. Updates to the Resource Connections database can be submitted to Brandon Cunningham at brandon.cunningham@vdh.virginia.gov.
In collaboration with the Division of Informatics and Information Systems (DIIS), DDP has released a new interactive dashboard for Ryan White services. The dashboard is a map of service providers throughout the Commonwealth. It allows users to filter providers by region or service type (part A, B, C, or D). Pop out maps are provided on the map to provide detailed views of areas with higher provider density. You can find the interactive map on the Ryan White Resources page. The webpage includes a description of the different Ryan White parts beneath the map. Please note that this dashboard does not take the place of a service directory and does not contain detailed contact information for agencies. If you see corrections or changes needed to the dashboard, please contact Rivkah Meder at rebecca.meder@vdh.virginia.gov. Updates to the Resource Connections database can be submitted to Brandon Cunningham at brandon.cunningham@vdh.virginia.gov.
Additionally, DDP has relaunched the mpox data dashboard. This dashboard is separate from the Monthly Surveillance Report. This will allow for a closer look at mpox data and will get updated weekly, not monthly.
Changes to National Clinician Consultation Center Hotline
The National Clinician Consultation Center (NCCC) now has one phone number for all programs, including:
-
- PrEP
- NPEP
- Hepatitis C
- Substance Use
- HIV/AIDS
- Perinatal HIV/AIDS
NCCC Hotline Number: 844-ASK-NCCC or 844-275-6222
Hotline hours are Monday through Friday from 11 a.m. to 7 p.m. ET, excluding holidays.
For now, if you call the previous numbers, you will automatically be forwarded to the new line.
Please note that Perinatal HIV/AIDS consultations will continue to be available 24 hours, seven days a week.
New Partnership Expands Access to Treatment for CHR Clients
The Comprehensive Harm Reduction (CHR) programs in the Lenowisco and Cumberland Plateau Health Districts are excited to announce a new partnership with Revida Recovery Centers to provide Medication-Assisted Treatment (MAT) via telemedicine.
Through this initiative, CHR clients who express interest will receive a warm, supportive, and streamlined introduction to treatment:
-
- A public health nurse will explain the service.
- If the client chooses to participate, vital signs will be taken, enrollment paperwork completed, and a point-of-care urine drug screen performed onsite.
- Specimens will be sent to Labcorp for confirmation testing.
- The client will then meet— in our telemedicine room—with an addiction specialist from Revida Recovery.
This approach eliminates the stress of a first appointment in an unfamiliar setting and ensures CHR staff is right there to offer encouragement. We know that many clients receive referrals but never take that next step—by bridging the gap with onsite support, we hope to make the path to recovery less intimidating and more accessible.
DDP is awarded funding for a Harm Reduction Program with Pregnant Women
The Opioid Abatement Authority (OAA) awarded $631,420 per year for a three-year pilot project to support women who are pregnant and using drugs. Programs will center on patient navigation and case management for participants. Pilot sites will work closely with Title X programs in local health departments and Contraceptive Access Initiative funded agencies. Over-the-counter birth control and condoms are available to CHR female participants who do not wish to become pregnant until they can be linked to a provider for prescription birth control pills or a longer lasting birth control method the woman might choose.
This project was born out of a pilot syphilis testing program for women of childbearing years at CHR sites. In that project, if a woman tested positive for syphilis, she was given a pregnancy test. CHR sites realized that they were serving more pregnant women than they thought. This pilot was created to link women to prenatal care and follow along services after birth. Follow-along services will last until the child turns two.
Several entities contributed to the development of this strategy, including:
-
- VDH’s Office or Family Health Services,
- Department of Behavioral Health and Developmental Services,
- Department of Medical Assistance Services,
- Department of Social Services,
- local health departments,
- Child Protection Services (CPS),
- CHR Site Staff, and
- pregnant mothers who are/were using drugs.
If successful, rates of opioid abstinence syndrome will decrease, women who enter treatment will stay in treatment after the child is born, women will deliver healthy babies, and the mother will be connected to needed resources for both her and her baby.
More information will be shared when it is available.
Clinical Guidelines Update: Advancing Perinatal HIV Care
On June 12, 2025, the U.S. Department of Health and Human Services (HHS) Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission has updated the recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the Unites States. The new updates to perinatal HIV care guidelines reflect the latest data and refining clinical practices.
Some key updates are summarized below:
Pregnancy and Postpartum HIV Testing and Identification of Perinatal and Postnatal Exposure
When determining the timing of repeat HIV testing in the third trimester, some clinicians conduct testing at or around 28 weeks of gestation together with the recommended timing of syphilis testing. This limits the number of blood draws and allows adequate time for syphilis treatment and congenital syphilis prophylaxis. Some clinicians also conduct a third test for HIV at the time of delivery hospitalization admission.
HIV Testing during Labor When HIV Status is Unknown
When there are plans to breastfeed, the panel strongly advises against initiating breastfeeding given the high risk of perinatal transmission. Breast milk should be expressed and stored appropriately. It should not be used for infant feeding unless all supplemental HIV test results are reviewed and determined to be negative.
Clinical Management of PrEP Use during Periconception, Antepartum, and Postpartum Periods
When prescribing PrEP, clinicians should confirm that no acute, potential exposure to HIV has occurred in the past 72 hours. In such cases, nPEP should be administered for 28 days prior to initiating PrEP.
Pre-Pregnancy Counseling and Partner Care
Assess knowledge about partner HIV status and the need to screen partner(s) for HIV and sexually transmitted infections. Provide testing or refer to services as needed. Discuss whether HIV status has been disclosed to sexual partner(s). Discuss options for PrEP when indicated.
Recommendations for the Use of Antiretroviral (ART) Drugs
If ART is not already being used during pregnancies impacted by HIV, ART should be initiated as early in pregnancy as possible, regardless of HIV RNA level or CD4 T lymphocyte cell count. This is to maximize health and prevent perinatal HIV transmission and sexual transmission.
During pregnancy, when there is a lack of experience with ART and no previous use of long-acting cabotegravir (CAB-LA) as pre-exposure prophylaxis (PrEP), Preferred regimens consist of the integrase strand transfer inhibitors (INSTIs) dolutegravir (DTG) or bictegravir (BIC) plus a tenofovir-containing dual–nucleoside reverse transcriptase inhibitor combination.
Abacavir (ABC) is also classified as an Alternative ARV drug for use in pregnancy.
These changes will help providers to deliver evidence-based care to pregnant women and infants. Visit HIV.gov to view all of the updates/revisions.
For more information, please contact Jasmine Christine Ford, HIV Care Services Clinical Coordinator, at jasmine.ford@vdh.virginia.gov. You may also contact Safere Diawara, HIV Care Services Manager of Clinical and Data Administration, at safere.diawara@vdh.virginia.gov.
“Gas Station Heroin”
U.S. poison centers have reported an increase in calls related to “tianeptine.” Tianeptine, also known as “gas station heroin,” is an antidepressant used in some Latin American, Asian, and European countries. Like opioids, tianeptine binds to opioid receptors in the brain. In the United States, it is not approved by the U.S. Food and Drug Administration (FDA) for human use and is not regulated under the Controlled Substances Act. In countries where tianeptine is prescribed, dosage ranges from 30 to 50 mg/daily. In the U.S., those who use tianeptine report much higher doses, on average approximately 1,500 mg/daily, with some reporting use in excess of 4,000 mg/daily. It is consumed orally, injected, or inhaled.
Tianeptine can be easily purchased in gas stations and online. It is typical sold as a nootropic (chemical to improve cognitive functioning), dietary supplement, or research chemical, with brand names such as “Pegasus,” “ZaZa Red,” “Tianna,” “Tianaa,” or “TD Red.” It is often sold as a powder or in tablet form. The Drug Enforcement Administration (DEA) reports law enforcement has seized tianeptine in the form of counterfeit pharmaceutical pills.
In high doses, tianeptine has opioid-like effects, and can lead to opioid-like dependance and withdrawal. Signs and symptoms can include:
-
- Fast heart rate,
- High blood pressure
- Nausea and vomiting
- Irritability or fatigue
- Blue lips or skin, small pupils, unresponsiveness
- Respiratory depression
First responders have reported that naloxone effectively reverses respiratory depression similar to opioid overdose. Withdrawal symptoms include:
-
- Gastrointestinal complaints
- Agitation and anxiety
- Muscle spasms
- Fast heart rate
- High blood pressure
- Tremors
Users have reported onset of severe withdrawal symptoms within only a few hours after use. The assumption that tianeptine can treat opioid use disorder among opioid users is false.
Recommendations
-
- Make naloxone available to the public and encourage its use when someone’s respiration is depressed, even when there are no signs of opioid use.
- Consider messages informing the public that just because a product is easy to obtain, does not mean that it is approved by the FDA or safe.
- In Virginia, the Blue Ridge Poison Center is available 24/7 to assist both the public, as well as clinicians seeking consultation. (Public: 1-800-222-1222; Healthcare professional hotline: 1-800-451-1428)
- Health care providers can submit adverse tianeptine effects to the FDA’s MedWatch reporting system.
Resources

DoxyPEP and PrEP Advertising
DDP is running Greater Than digital ads for both DoxyPEP and PrEP throughout Virginia during August.
The ads are being focused in areas where new cases of HIV, syphilis, and congenital syphilis have been diagnosed. Any community partners that offer DoxyPEP or PrEP services and are listed in national locators may receive an increase in inquiries or contact from clients as a result of these efforts. Communications have already been distributed to our local health departments.
For any questions about our digital ad efforts, or for copies of the assets being used for your use on social media and other digital platforms, please contact Chris Barnett, Public Relations Coordinator, at christopher.barnett@vdh.virginia.gov.
New Content from the National STD Curriculum
The National STD Curriculum is a free educational website from the University of Washington STD Prevention Training Center. They have recently added new podcast episodes, mini-lectures, and lessons to their available free resources. Find links to their pages below to access their content and continue your education now.
Personnel Announcements
No DDP personnel updates for August 2025.
![]()
Subscription Preferences
Join the DDP E-bulletin Mailing List
Manage Preferences | Subscriber Help
