New Resource Connections (The RC) Site Launched
 The new site for Resource Connections has launched! With the relaunch we've also rebranded it to call it “The RC” in short. Call it whichever you prefer – we'll know what you mean!
The new site for Resource Connections has launched! With the relaunch we've also rebranded it to call it “The RC” in short. Call it whichever you prefer – we'll know what you mean!
The old site is no longer, but the old URL now automatically redirects to the new. The new URL for The RC is https://therc.vdh.virginia.gov. You’ll find that the new website has a brand-new interface and design. Additionally, the new site uses the Connect 211 functionality like Virginia 211.
VDH staff regularly review and update agency data to ensure accuracy. Most agencies that have an HIV prevention or care funding contract have a requirement in those contracts that they must update their agency’s data in The RC on an annual basis at minimum.
Agencies will not be able to update their own information at this time. It is estimated that this functionality will be restored within the next six months. We will communicate when agency data self-service is restored. A training will also be offered at that time. Until then, any agencies that have outdated information on The RC should request their agency information/data updates to the Disease Prevention Hotline at hiv-stdhotline@vdh.virginia.gov.
11th Annual Virginia Ryan White Case Management Summit
The MidAtlantic AIDS Education and Training Center (MAAETC) local partner site, Virginia Commonwealth University (VCU), will be partnering with VDH, HIV case managers, and the Ryan White Program to host the 11th Annual Virginia Ryan White Case Management Summit.
The summit will be held, virtually, on Tuesday April 21, from 10 a.m. until 4 p.m., and Wednesday, April 22, from 10 a.m. until 3 p.m.
The summit is open to all medical and non-medical case managers, HOPWA case managers, service navigators, community health workers, and others providing case management services to Virginians with HIV.
The theme of this year’s summit is Empowering New Pathways: The Work Continues. Session topics will include:
-
- HIV/AIDS in 2026
- Partnering through internships and interprofessional education to support the next generation of the HIV workforce
- The use of technology in HIV case management
- Anger, de-escalation, and resiliency in case management
- Supporting health aging through nutrition
- Regional breakout sessions
- And more!
For questions, contact Rob Rodney, Director of the HIV Education Program at VCU, at robert.rodney@vcuhealth.org.
VDH Integrated HIV Prevention & Care Town Hall
VDH DDP is holding a virtual VDH Integrated HIV Prevention and Care Town Hall. The discussion will tie direction into the Virginia Integrated HIV Services Plan.
The town hall will be held Wednesday, March 4, 2026, from 6 p.m. until 7 p.m. It is a virtual (online) meeting with a call-in option.
Contact Olivia Allison at olivia.allison@vdh.virginia.gov with questions or for more information.
National Women and Girl's HIV/AIDS Awareness Day
National Women and Girl’s HIV/AIDS Awareness Day (NWGHAAD) is on March 10, 2026. This year marks the 20th annual observance of NWGHAAD.
You can help raise awareness of this observance by:
-
- Using messaging and graphics from the following organizations:
- Sharing VDH & DDP social media posts
- Using the hashtag #NWGHAAD on any relevant posts you create of your own
- Access information on HIV and women and hold HIV outreach and testing events for women for NWGHAAD
Learn more about NWGHAAD on the WomensHealth.gov NWGHAAD page.
National LGBTQ Health Awareness Week
National LGBTQ Health Awareness Week is coming up March 16th through the 20th. The week calls for healthcare professionals, advocates, and community leaders to collaborate to address and provide LGBTQ healthcare and health equity.
Visit the healthlgbtq.org website to learn more about the week and find resources and graphics to share on your website or social media.
LGBTQ Equity in HIV Prevention and Treatment Series
The National Coalition for LGBTQ Health offers a multi-module series in collaboration with HealthHIV to strength capacity to provide care to LGBTQ persons.
The module is a FREE self-paced CME accredited program composed of five, 30-minute module addressing strategies to reduce HIV disparities among LGBTQ populations.
The target audience for the modules include:
-
- Primary care providers
- HIV care providers
- LGBTQ health providers
- General practitioners
- Nurses
- Pharmacists
- Patients and caregivers
All modules are certified, and participants can earn up to 3.75 credits/contact hours (CME, MOC, AAPA, ANCC, CPE, ASWB, APA) for completion of all program components.
Personnel Announcements
We’re Hiring!
DDP is hiring the Director of HIV Care Services.
Please apply or share with anyone who may be interested.
![]()
Subscription Preferences
Join the DDP E-bulletin Mailing List
Manage Preferences | Subscriber Help




 The Centers for Disease Control and Prevention (CDC) has announced another 12-month extension to the Strengthening STD Prevention and Control for Health Departments (STD PCHD) grant. The extension will cover the period of March 1, 2026, through February 28, 2027.
The Centers for Disease Control and Prevention (CDC) has announced another 12-month extension to the Strengthening STD Prevention and Control for Health Departments (STD PCHD) grant. The extension will cover the period of March 1, 2026, through February 28, 2027.










 Mpox cases continue to occur primarily in men. Mpox also disproportionately affects communities of color. Mpox cases have occurred in all five state health regions, with the greatest number of cases reported from Virginia’s Northern and Central health planning regions (82%).
Mpox cases continue to occur primarily in men. Mpox also disproportionately affects communities of color. Mpox cases have occurred in all five state health regions, with the greatest number of cases reported from Virginia’s Northern and Central health planning regions (82%).



 On September 30, 2025, DDP received a surprising notice of award (NoA) for the HIV Medical Monitoring Project (MMP). Prior funding for MMP ended on May 31, 2025, and we were told the grant would not be renewed. Therefore, DDP ended the program and MMP staff were unfortunately laid off. Due to the unexpected nature of this new award, the back date of the project period to 6/1/25, and current federal government shut down, the remainder of the first year, or grant year 1, will be used to recruit staff and re-establish the program. DDP is awaiting further guidance from CDC, which will occur after the resolution of the federal government shutdown.
On September 30, 2025, DDP received a surprising notice of award (NoA) for the HIV Medical Monitoring Project (MMP). Prior funding for MMP ended on May 31, 2025, and we were told the grant would not be renewed. Therefore, DDP ended the program and MMP staff were unfortunately laid off. Due to the unexpected nature of this new award, the back date of the project period to 6/1/25, and current federal government shut down, the remainder of the first year, or grant year 1, will be used to recruit staff and re-establish the program. DDP is awaiting further guidance from CDC, which will occur after the resolution of the federal government shutdown. DIS (Disease Intervention Specialist) Recognition Day was Friday, October 3. This special day is observed nationally each year to promote the special work that our DIS do within our communities. The National Coalition for STD Directors, VDH, and DDP had blog postings for the occasion and promoted or shared posts and graphics on social media.
DIS (Disease Intervention Specialist) Recognition Day was Friday, October 3. This special day is observed nationally each year to promote the special work that our DIS do within our communities. The National Coalition for STD Directors, VDH, and DDP had blog postings for the occasion and promoted or shared posts and graphics on social media. The Resource Connections website is being relaunched with a fresh new look and a new name — The RC: Resource Connections — on the Connect 211 platform! Along with the updated design, the site will feature a new logo and URL.
The Resource Connections website is being relaunched with a fresh new look and a new name — The RC: Resource Connections — on the Connect 211 platform! Along with the updated design, the site will feature a new logo and URL.

 The Virginia Community HIV Planning Group (CHPG) needs new members. The CHPG works with DDP to develop and monitor Virginia’s Integrated HIV Services Plan. Their main goal is to end the HIV epidemic and improve the health of people with HIV. The Plan will guide DDP work over the next five years.
The Virginia Community HIV Planning Group (CHPG) needs new members. The CHPG works with DDP to develop and monitor Virginia’s Integrated HIV Services Plan. Their main goal is to end the HIV epidemic and improve the health of people with HIV. The Plan will guide DDP work over the next five years. Bulk condom orders are currently on hold while the Department of General Services negotiates with Global Protection to renew its contract with the Commonwealth of Virginia. This situation has extended beyond initial expectations, resulting in temporary shortages of several brands, including non-latex condom options, lubricant, and Trojan Magnum condoms.
Bulk condom orders are currently on hold while the Department of General Services negotiates with Global Protection to renew its contract with the Commonwealth of Virginia. This situation has extended beyond initial expectations, resulting in temporary shortages of several brands, including non-latex condom options, lubricant, and Trojan Magnum condoms.

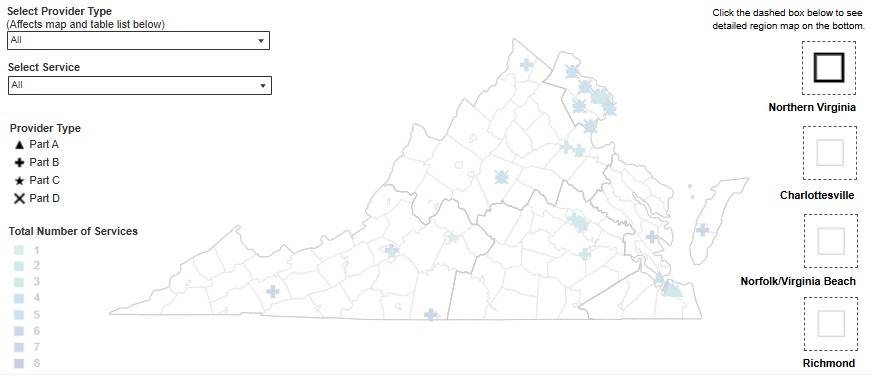 In collaboration with the Division of Informatics and Information Systems (DIIS), DDP has released a new interactive dashboard for Ryan White services. The dashboard is a map of service providers throughout the Commonwealth. It allows users to filter providers by region or service type (part A, B, C, or D). Pop out maps are provided on the map to provide detailed views of areas with higher provider density. You can find the interactive map on the
In collaboration with the Division of Informatics and Information Systems (DIIS), DDP has released a new interactive dashboard for Ryan White services. The dashboard is a map of service providers throughout the Commonwealth. It allows users to filter providers by region or service type (part A, B, C, or D). Pop out maps are provided on the map to provide detailed views of areas with higher provider density. You can find the interactive map on the 


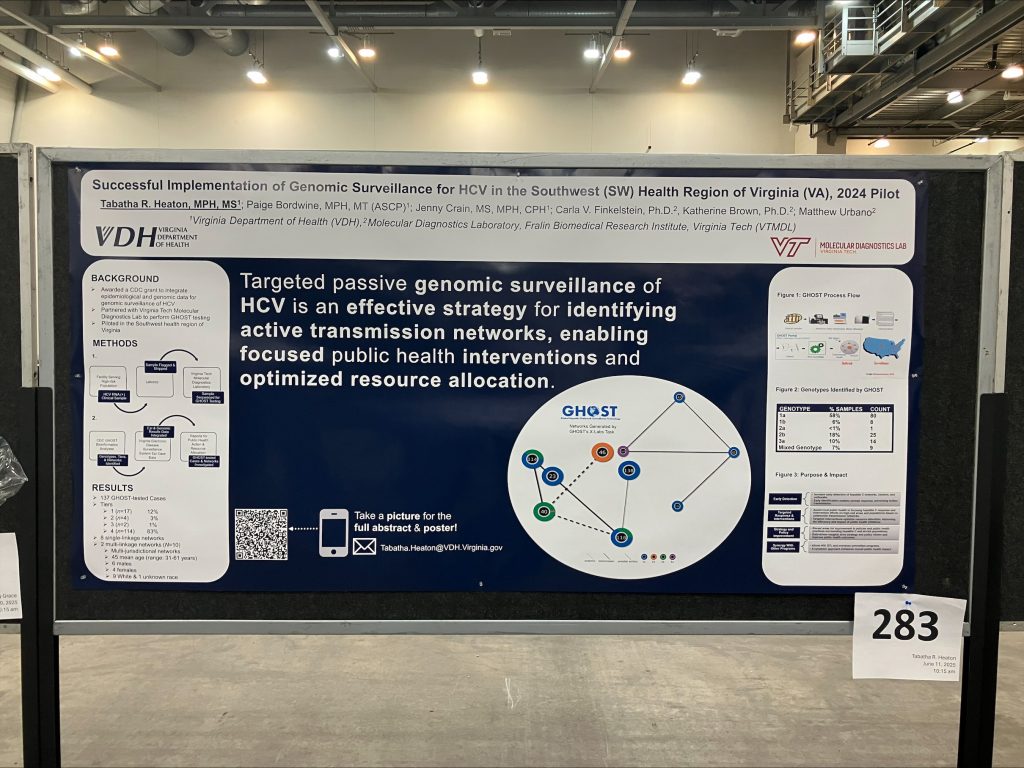 C Virus (HCV) Genomic Surveillance program in the Southwest health region. The program is intended to show effective integration of data for identifying transmission networks, specifically, genomic and epidemiological data. This approach aims to support targeted public health responses and interventions. By October 1, 2024, DDP analyzed 137 cases using the CDC-developed Global Hepatitis Outbreak Surveillance Technology (GHOST) bioinformatics system. It identified eight single-linkage clusters and two multi-linkage, jurisdiction-spanning clusters. Notably, mixed infections were detected within both multi-linkage clusters. This indicated recent transmission events.
C Virus (HCV) Genomic Surveillance program in the Southwest health region. The program is intended to show effective integration of data for identifying transmission networks, specifically, genomic and epidemiological data. This approach aims to support targeted public health responses and interventions. By October 1, 2024, DDP analyzed 137 cases using the CDC-developed Global Hepatitis Outbreak Surveillance Technology (GHOST) bioinformatics system. It identified eight single-linkage clusters and two multi-linkage, jurisdiction-spanning clusters. Notably, mixed infections were detected within both multi-linkage clusters. This indicated recent transmission events.

 The study is a large, long-term, state-by-state analysis published in the
The study is a large, long-term, state-by-state analysis published in the