On June 12, 2025, the U.S. Department of Health and Human Services (HHS) Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission has updated the recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the Unites States. The new updates to perinatal HIV care guidelines reflect the latest data and refining clinical practices.
Some key updates are summarized below:
Pregnancy and Postpartum HIV Testing and Identification of Perinatal and Postnatal Exposure
When determining the timing of repeat HIV testing in the third trimester, some clinicians conduct testing at or around 28 weeks of gestation together with the recommended timing of syphilis testing. This limits the number of blood draws and allows adequate time for syphilis treatment and congenital syphilis prophylaxis. Some clinicians also conduct a third test for HIV at the time of delivery hospitalization admission.
HIV Testing during Labor When HIV Status is Unknown
When there are plans to breastfeed, the panel strongly advises against initiating breastfeeding given the high risk of perinatal transmission. Breast milk should be expressed and stored appropriately. It should not be used for infant feeding unless all supplemental HIV test results are reviewed and determined to be negative.
Clinical Management of PrEP Use during Periconception, Antepartum, and Postpartum Periods
When prescribing PrEP, clinicians should confirm that no acute, potential exposure to HIV has occurred in the past 72 hours. In such cases, nPEP should be administered for 28 days prior to initiating PrEP.
Pre-Pregnancy Counseling and Partner Care
Assess knowledge about partner HIV status and the need to screen partner(s) for HIV and sexually transmitted infections. Provide testing or refer to services as needed. Discuss whether HIV status has been disclosed to sexual partner(s). Discuss options for PrEP when indicated.
Recommendations for the Use of Antiretroviral (ART) Drugs
If ART is not already being used during pregnancies impacted by HIV, ART should be initiated as early in pregnancy as possible, regardless of HIV RNA level or CD4 T lymphocyte cell count. This is to maximize health and prevent perinatal HIV transmission and sexual transmission.
During pregnancy, when there is a lack of experience with ART and no previous use of long-acting cabotegravir (CAB-LA) as pre-exposure prophylaxis (PrEP), Preferred regimens consist of the integrase strand transfer inhibitors (INSTIs) dolutegravir (DTG) or bictegravir (BIC) plus a tenofovir-containing dual–nucleoside reverse transcriptase inhibitor combination.
Abacavir (ABC) is also classified as an Alternative ARV drug for use in pregnancy.
These changes will help providers to deliver evidence-based care to pregnant women and infants. Visit HIV.gov to view all of the updates/revisions.
For more information, please contact Jasmine Christine Ford, HIV Care Services Clinical Coordinator, at jasmine.ford@vdh.virginia.gov. You may also contact Safere Diawara, HIV Care Services Manager of Clinical and Data Administration, at safere.diawara@vdh.virginia.gov.



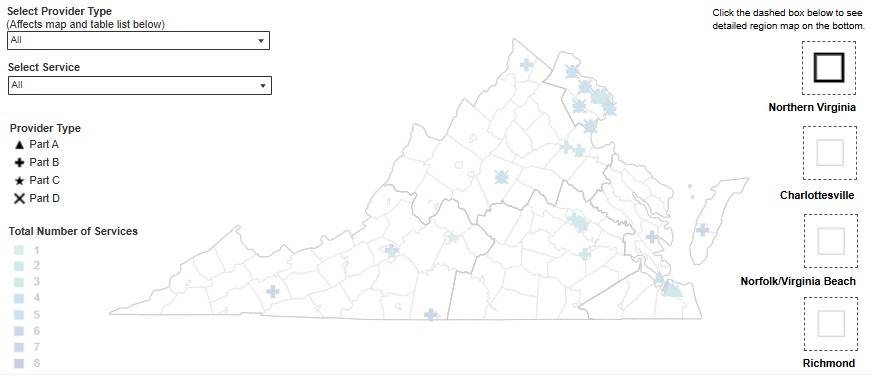 In collaboration with the Division of Informatics and Information Systems (DIIS), DDP has released a new interactive dashboard for Ryan White services. The dashboard is a map of service providers throughout the Commonwealth. It allows users to filter providers by region or service type (part A, B, C, or D). Pop out maps are provided on the map to provide detailed views of areas with higher provider density. You can find the interactive map on the
In collaboration with the Division of Informatics and Information Systems (DIIS), DDP has released a new interactive dashboard for Ryan White services. The dashboard is a map of service providers throughout the Commonwealth. It allows users to filter providers by region or service type (part A, B, C, or D). Pop out maps are provided on the map to provide detailed views of areas with higher provider density. You can find the interactive map on the 