COVID-19 Case and Vaccination Reports
The Richmond/Henrico Health Districts prepare a weekly report with the latest COVID-19 case data, including COVID testing encounters and positivity, cases, hospitalizations, fatalities, and vaccinations.
April 2023
- WEEKLY CASE REPORT, April 21, 2023 (PDF-10MB)
- WEEKLY CASE REPORT, April 14, 2023 (PDF-10MB)
- WEEKLY CASE REPORT, April 7, 2023 (PDF-10MB)
March 2023
- WEEKLY CASE REPORT, March 31, 2023 (PDF-10MB)
- WEEKLY CASE REPORT, March 24, 2023 (PDF-10MB)
- WEEKLY CASE REPORT, March 3, 2023 (PDF-8MB)
February 2023
- WEEKLY CASE REPORT, February 24, 2023 (PDF-2MB)
- WEEKLY CASE REPORT, February 17, 2023 (PDF-2MB)
- WEEKLY CASE REPORT, February 10, 2023 (PDF-2MB)
- WEEKLY CASE REPORT, February 3, 2023 (PDF-2MB)
January 2023
CASE REPORT ARCHIVES 2022
December 2022
- WEEKLY CASE REPORT, December 16, 2022
- WEEKLY CASE REPORT, December 9, 2022
- WEEKLY CASE REPORT, December 2, 2022
November 2022
- WEEKLY CASE REPORT, November 18, 2022
- WEEKLY CASE REPORT, November 11, 2022
- WEEKLY CASE REPORT, November 4, 2022
October 2022
- WEEKLY CASE REPORT, October 28, 2022
- WEEKLY CASE REPORT, October 21, 2022
- WEEKLY CASE REPORT, October 14, 2022
- WEEKLY CASE REPORT, October 7, 2022
September 2022
- WEEKLY CASE REPORT, September 30, 2022
- WEEKLY CASE REPORT, September 23, 2022
- WEEKLY CASE REPORT, September 15, 2022
- WEEKLY CASE REPORT, September 9, 2022
- WEEKLY CASE REPORT, September 2, 2022
August 2022
- WEEKLY CASE REPORT, August 26, 2022
- WEEKLY CASE REPORT, August 19, 2022
- WEEKLY CASE REPORT, August 12, 2022
- WEEKLY CASE REPORT, August 5, 2022
July 2022
- WEEKLY CASE REPORT, July 29, 2022
- WEEKLY CASE REPORT, July 22, 2022
- WEEKLY CASE REPORT, July 15, 2022
- MONTHLY CASE REPORT, July 7, 2022
June 2022
- WEEKLY CASE REPORT, June 30, 2022
- WEEKLY CASE REPORT, June 20, 2022
- WEEKLY CASE REPORT, June 13, 2022
- MONTHLY CASE REPORT, June 6, 2022
May 2022
- WEEKLY CASE REPORT, May 30, 2022
- WEEKLY CASE REPORT, May 23, 2022
- WEEKLY CASE REPORT, May 16, 2022
- WEEKLY CASE REPORT, May 9, 2022
- WEEKLY CASE REPORT, May 2, 2022
April 2022
- WEEKLY CASE REPORT, April 25, 2022
- WEEKLY CASE REPORT, April 18, 2022
- WEEKLY CASE REPORT, April 11, 2022
- MONTHLY CASE REPORT, April 5, 2022
March 2022
- WEEKLY CASE REPORT, March 28, 2022
- WEEKLY CASE REPORT, March 21, 2022
- WEEKLY CASE REPORT, March 14, 2022
- WEEKLY CASE REPORT, March 7, 2022
February 2022
January 2022
CASE REPORT ARCHIVES 2021
December 2021
November 2021
- MONTHLY CASE REPORT, Oct 18-Dec 5, 2021
- WEEKLY CASE REPORT, Nov 29 2021
- WEEKLY CASE REPORT, Nov 22 2021
- WEEKLY CASE REPORT, Nov 15 2021
- WEEKLY CASE REPORT, Nov 8 2021
- WEEKLY CASE REPORT, Nov 1 2021
October 2021
- WEEKLY CASE REPORT, Oct 19 – 25, 2021
- MONTHLY CASE REPORT, Sept 21 – Oct 18, 2021
- WEEKLY CASE REPORT, Oct 10 – 14, 2021
- WEEKLY CASE REPORT, Oct 4 – 10, 2021
September 2021
- WEEKLY CASE REPORT, Sept 20 – 27, 2021
- MONTHLY CASE REPORT, Aug 23 – Sept 19, 2021
- WEEKLY CASE REPORT, Sept 7 – 13, 2021
- WEEKLY CASE REPORT, Aug 31 – Sept 6, 2021
August 2021
- WEEKLY CASE REPORT, Aug 23 - Aug 30, 2021
- MONTHLY CASE REPORT, July 26- August 22, 2021
- WEEKLY CASE REPORT, Aug 9 - Aug 16, 2021
- WEEKLY CASE REPORT, Aug 2 - Aug 9, 2021
- WEEKLY CASE REPORT, July 26 - Aug 2, 2021
July 2021
- MONTHLY CASE REPORT, June 29 - July 25, 2021
- WEEKLY CASE REPORT, July 12 - July 19, 2021
- WEEKLY CASE REPORT, July 5 - July 12, 2021
- WEEKLY CASE REPORT, June 28 - July 5, 2021
June 2021
- MONTHLY CASE REPORT, June 1 - June 28, 2021
- WEEKLY CASE REPORT, June 14 - June 21, 2021
- WEEKLY CASE REPORT, June 7 - June 14, 2021
- WEEKLY CASE REPORT, May 31 - June 7, 2021
May 2021
- MONTHLY CASE REPORT, MAY 1 - MAY 31, 2021
- WEEKLY CASE REPORT, MAY 17 - MAY 24, 2021
- WEEKLY CASE REPORT, MAY 10 - MAY 17, 2021
- WEEKLY CASE REPORT, MAY 3 - MAY 10, 2021
April 2021
- MONTHLY CASE REPORT, APR 1 - MAY 3, 2021
- WEEKLY CASE REPORT, MON APR 19 - SUN APR 26, 2021
- WEEKLY CASE REPORT, MON APR 12 - SUN APR 18, 2021
- WEEKLY CASE REPORT, MON APR 5 - SUN APR 11, 2021
March 2021
- MONTHLY CASE REPORT, MAR 1 - APR 4, 2021
- WEEKLY CASE REPORT, MON MAR 22 - SUN MAR 28, 2021
- WEEKLY CASE REPORT, MON MAR 15 - SUN MAR 21, 2021
- WEEKLY CASE REPORT, MON MAR 8 - SUN MAR 14, 2021
- WEEKLY CASE REPORT, MON MAR 1 - SUN MAR 7, 2021
February 2021
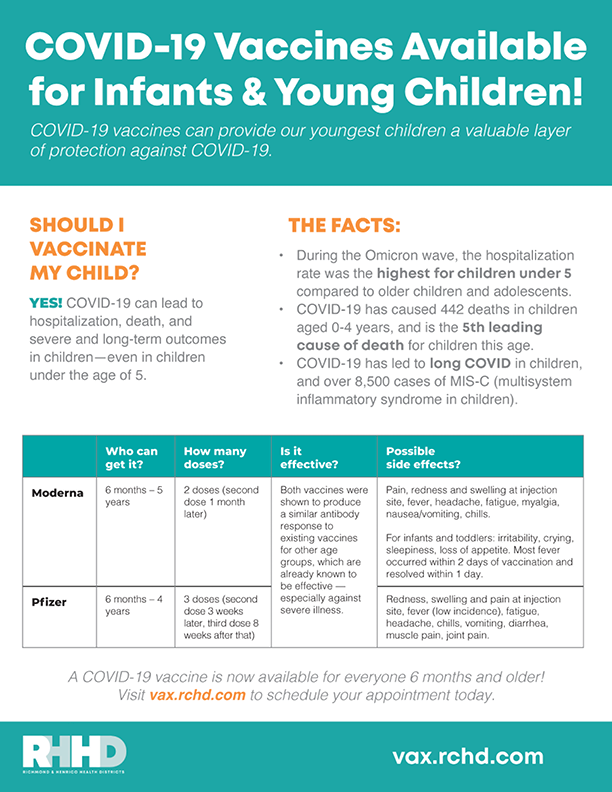
IMPORTANT INFO! FAQs ABOUT THE COVID-19 VACCINE FOR INFANTS AND YOUNG CHILDREN
[Vaccines for Young Children (PDF-241KB)]